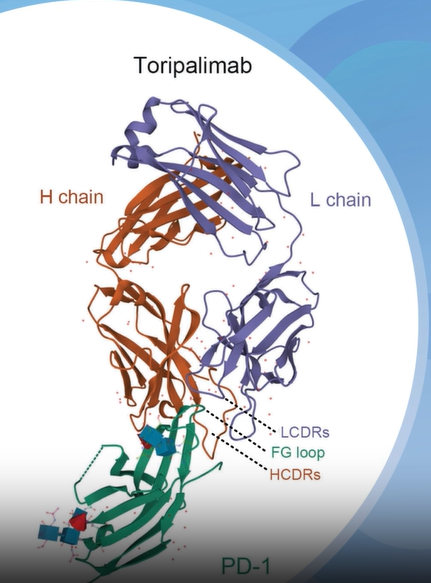
Toripalimab
References
- FDA: US Food and Drug Administration .
- EMA: European Medicines Evaluation Agency .
- ANVISA: Brazilian Health Regulatory Agency .
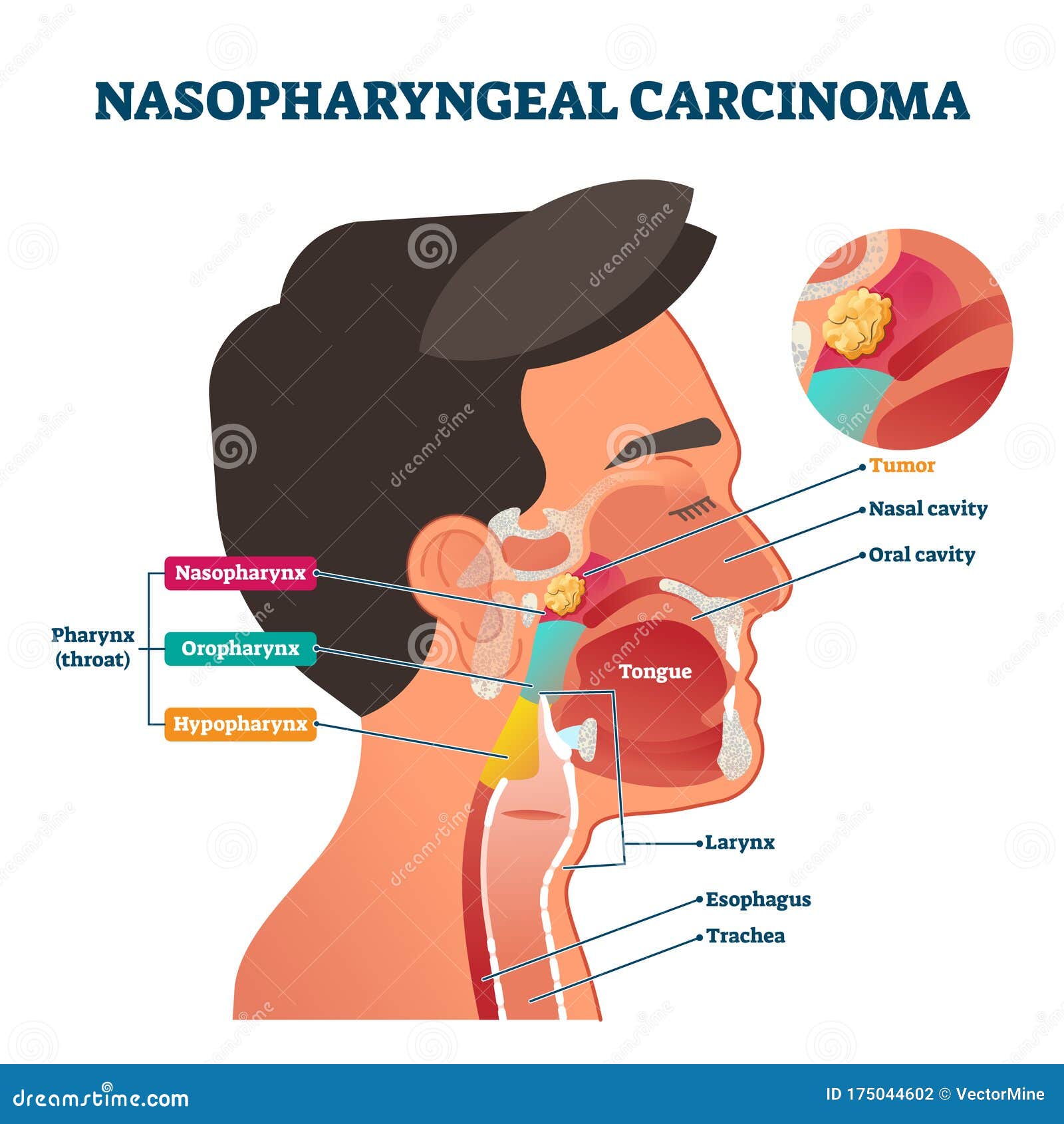
- R/M NPC: Recurrent Locally Advanced or Metastatic Nasopharyngeal Cancer .
- CI: Confidence interval.
- HR: Hazard rate.
References:
- New Drug Therapy Approvals 2023 – FDA . Available at: https://www.fda.gov/media/175253/download . Accessed on: 11/25/2024.
- Junshi Biosciences Announces Positive Opinion from the European Medicines Agency's CHMP For Toripalimab . Available at: https://www.globenewswire.com/en/news-release/2024/07/26/2919683/0/en/Junshi-Biosciences-Announces-Positive-Opinion-from-the-European-Medicines-Agency-s-CHMP-For-Toripalimab.html. Accessed on: 25/11/2024.
- ANVISA approval reference – to be made available after approval.
- Zytorvi® package insert . Available at: (link pending). Accessed on: (date pending)