DOSAGE AND ADMINISTRATION FOR FIRST-LINE TREATMENT
DOSAGE AND ADMINISTRATION FOR FIRST-LINE TREATMENT
RECOMMENDED DOSAGE
ZYTORVI® is indicated, in combination with cisplatin and gemcitabine, for the first-line treatment of adults with locally advanced, recurrent or metastatic NPC. See the Prescribing Information for cisplatin and gemcitabine for recommended dosing information.
In JUPITER-02, Zytorvi ® was administered:
ZYTORVI® was administered without chemotherapy after the first 6 cycles
Administration
-
ZYTORVI IV Infusion Details:
-
Administer the diluted solution intravenously through the infusion pump using an in-line aseptic filter (0.2 or 0.22 micron)
-
First infusion: Infuse for at least 60 minutes
-
Subsequent infusions: If no infusion-related reactions occur during the first infusion, subsequent infusions may be administered within 30 minutes.
-
Do not administer other medications through the same IV line.
-
When administered on the same day as chemotherapy, ZYTORVI® should be administered prior to chemotherapy.
-
Please refer to the Prescribing Information for cisplatin and gemcitabine for recommended dosage information.
-
IV=intravenous; NPC=nasopharyngeal carcinoma; R/M NPC=recurrent locally advanced/metastatic nasopharyngeal carcinoma.
DOSAGE AND ADMINISTRATION FOR SUBSEQUENT TREATMENT

ZYTORVI® is administered in a chemotherapy-free regimen
Patients should receive ZYTORVI® until disease progression or unacceptable toxicity.
Administration
-
First infusion: Infuse for at least 60 minutes
-
Subsequent infusions: If no infusion-related reactions occur during the first infusion, subsequent infusions may be administered within 30 minutes.
-
Do not administer other medications through the same IV line.
IV=intravenous
RECOMMENDED DOSE MODIFICATIONS FOR ADVERSE REACTIONS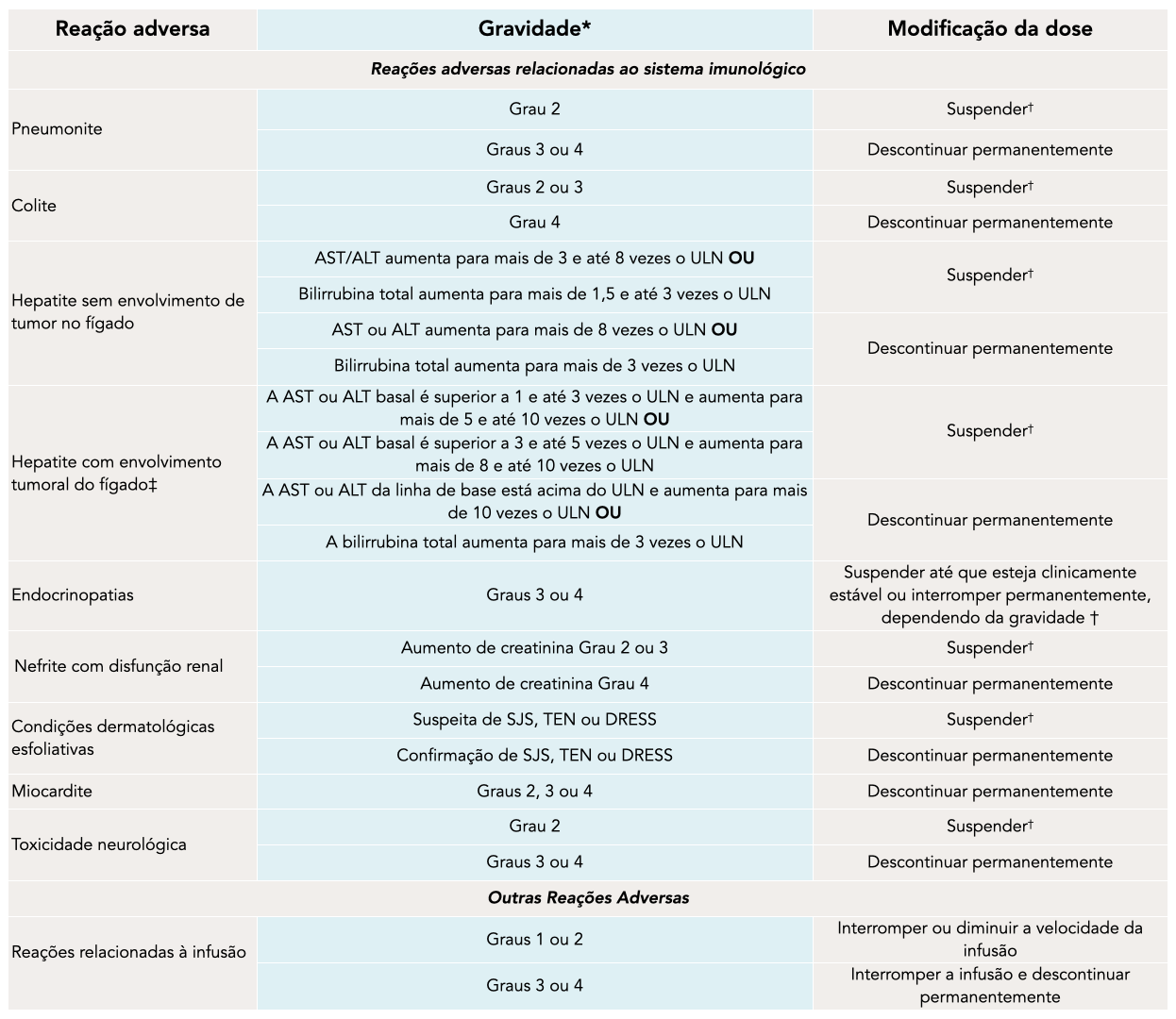
*Based on the National Cancer Institute (NCI) Common Terminology for Adverse Events (CTCAE) version 5.0.1
† Resume ZYTORVI® in patients with complete or partial resolution to Grade 0 or 1 after corticosteroid taper. Permanently discontinue if complete or partial resolution is not achieved within 12 weeks of starting steroids or if prednisone cannot be reduced to 10 mg daily or less (or equivalent) within 12 weeks of starting steroids.1
‡ If AST and ALT are less than or equal to the ULN at baseline in patients with liver involvement, withhold or permanently discontinue ZYTORVI® based on recommendations for hepatitis without liver involvement.1
ALT=alanine aminotransferase; AST=aspartate aminotransferase; DRESS=drug rash with eosinophilia and systemic symptoms; SJS=Stevens-Johnson syndrome; TEN=toxic epidermal necrolysis; ULN=upper limit of normal.
PREPARATION AND STORAGE
Preparation for IV infusion
-
Visually inspect the solution for particulate matter and discoloration. The solution is clear to slightly opalescent, colorless to slightly yellow. Discard the vial if visible particulate matter is observed.
-
Withdraw the required volume of ZYTORVI® and slowly inject into a 100 mL or 250 mL infusion bag containing 0.9% Sodium Chloride Injection, USP. Mix the diluted solution by gentle inversion. Do not shake. The final concentration of the diluted solution should be between 1 mg/mL and 3 mg/mL.
-
ZYTORVI® is compatible with polypropylene infusion bags and infusion sets with 0.2 or 0.22 micron in-line filter
-
Discard any unused portion left in the jar.
IV=intravenous.
Storage of diluted infusion solution
Zytorvi® does not contain a preservative. If the diluted solution is not administered immediately, store:
-
At room temperature, 20°C to 25°C (68°F to 77°F), for a maximum of 8 hours from the time of dilution until completion of infusion. Discard diluted solution stored at room temperature after 8 hours.
OR
-
Refrigerated at 2°C to 8°C (36°F to 46°F) for a maximum of 24 hours from time of dilution until completion of infusion. If refrigerated, allow diluted solution to reach room temperature prior to administration. Discard refrigerated diluted solution after 24 hours.
-
Do not freeze.
Reference: 1. ZYTORVI® (toripalimab-tpzi) Prescribing Information. Hyderabad, India: Dr. Reddy's Laboratories, Inc.