Jupiter-02 study
JUPITER-02: Evaluates the efficacy of toripalimab in first-line treatment for R/M NPC
JUPITER-02 study design
JUPITER-02, an international, multicenter, randomized, double-blind, single-region, placebo-controlled phase 3 study was designed to investigate the efficacy of ZYTORVI ® in combination with cisplatin and gemcitabine . It enrolled a group of 289 adult patients with R/M NPC who had not received prior systemic chemotherapy for recurrent or metastatic disease.¹† 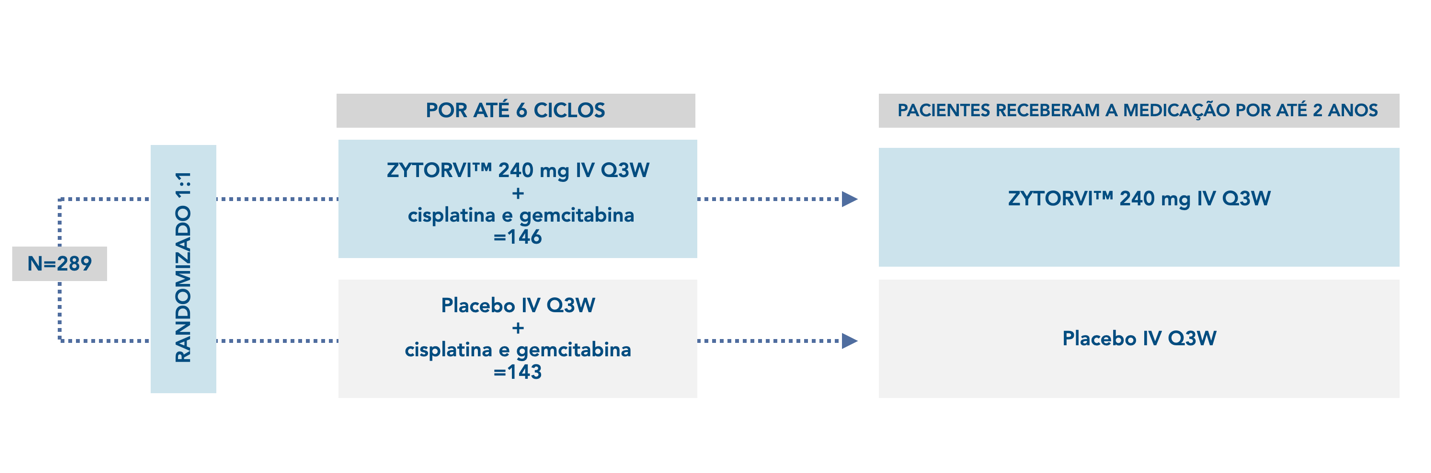
Treatment with ZYTORVI ® or placebo continued until disease progression according to RECIST v1.1, unacceptable toxicity, or a maximum of 2 years. The primary endpoint was BIRC-assessed PFS (progression-free survival) according to RECIST v1.1 criteria. Secondary endpoints were BIRC-assessed ORR, BIRC-assessed DOR, and OS. 1,2
*The JUPITER-02 study was conducted at 35 centers located in NPC-endemic regions, including mainland China, Taiwan, and Singapore.2
†Patients with recurrent NPC after curative-intent treatment were required to have an interval of at least 6 months between the last dose of radiotherapy or chemotherapy and recurrence. Patients with autoimmune disease, except stable hypothyroidism or type 1 diabetes, and patients requiring systemic immunosuppression were not eligible. 1
R/M NPC = Recurrent Locally Advanced or Metastatic Nasopharyngeal Cancer ; BIRC = Blinded independent review committee; DOR = Duration of response; IV = Intravenous; ORR = Overall response rate; OS = Overall survival; PFS = Progression-free survival; Q3W = Every 3 weeks; RECIST = Response evaluation criteria in solid tumors.
Key Eligibility and Exclusion Criteria²
Eligibility Criteria:
-
Primary metastatic or recurrent locally advanced NPC after curative intent therapy;
-
Initial treatment ( naive ) for R/M NPC;
-
ECOG PS 0-1;
-
18-75 years old;
-
Measurable disease according to RECIST v1.1.
Exclusion Criteria:
-
History of severe hypersensitivity reactions to any monoclonal antibody, cisplatin or gemcitabine, or to any ingredient in Zytorvi® ;
-
Active or untreated central nervous system metastases;
-
Previous treatment with a monoclonal antibody targeting PD-1/PD-L1/CTLA4 ;
-
History of bone marrow or solid organ transplant;
-
History of autoimmune disease specified in the protocol.
Demographics:
-
Median Age : 48 years (range: 19 to 72) - 4.8% ≥ 65 years
-
Sex : 83% men
-
Ethnicity : 100% Asian
-
ECOG Performance Status (PS) : 57% with PS of 0
-
Metastasis : 86% had metastasis
Histological Subtypes:
-
98% non-keratinizing
-
1% keratinizing squamous cell carcinoma
-
1% unidentified
Pre-treatment:
-
Approximately 59% received ≥1 prior systemic therapy for locally advanced disease
-
60% received prior radiotherapy
CTLA4 = cytotoxic T-cell-associated protein 4; ECOGPS = Eastern Cooperative Oncology Group performance status; PD-L1 = programmed death ligand 1.
Progression-free survival: interim analysis (primary outcome) 4.5
ZYTORVI®+ chemotherapy significantly reduced the risk of progression or death 1
BIRC-assessed mPFS of 11.7 months versus 8 months with placebo + chemotherapy 1.2
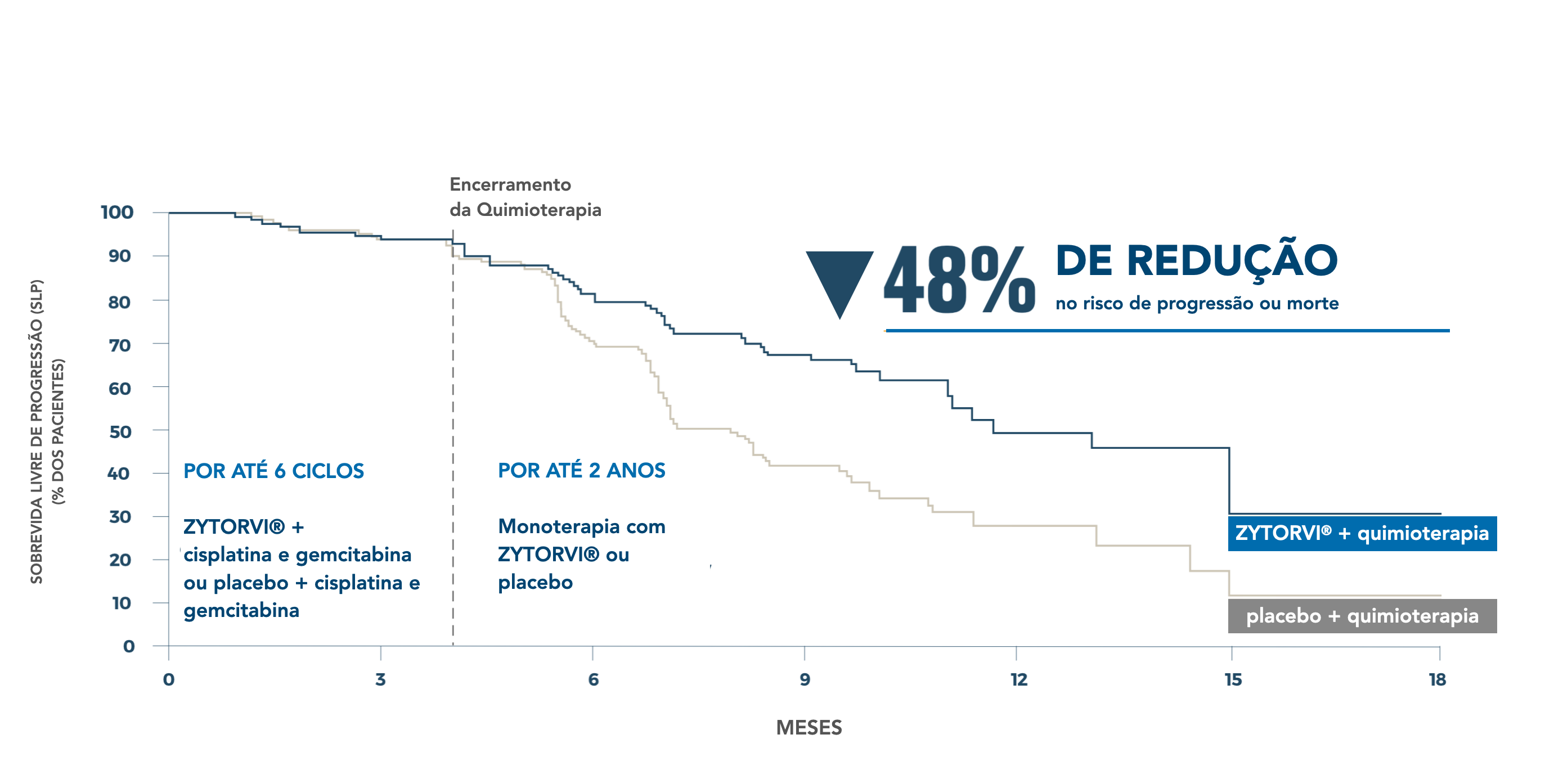
No. of events
(%)
49
(34%)
ZYTORVI®
+ chemotherapy
79
(55%)
Placebo
+ chemotherapy
Median PFS
months (95% CI)
11.7
(11.0-NE)
ZYTORVI®
+ chemotherapy
8
(7.0-9.5)
Placebo
+ chemotherapy
PFS HR (95% CI)† : 0.52 (0.37-0.73)
P-value ‡: 0.001
*Cutoff date for interim analysis was May 30, 2020.¹
†Based on stratified Cox proportional hazards model using stratification factors at randomization, ECOG performance status, and disease stage.¹
‡Two-sided P value, based on stratified log-rank test, compared with an alpha threshold of 0.010.¹
Progression-free survival: final analysis 4.5
ZYTORV ® : Chemotherapy offered lasting protection against progression4,58
Final PFS by BIRC according to RECIST v1.1
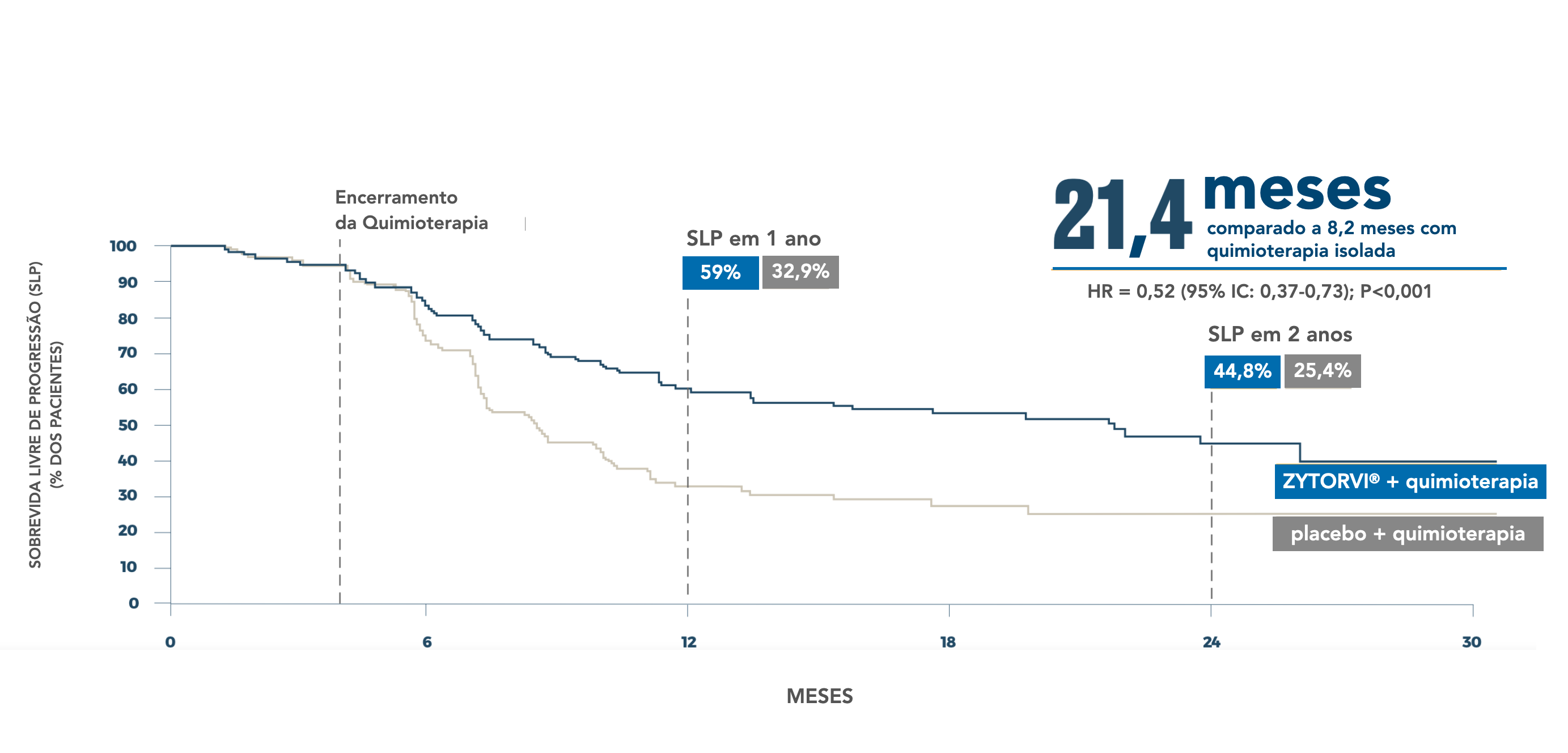
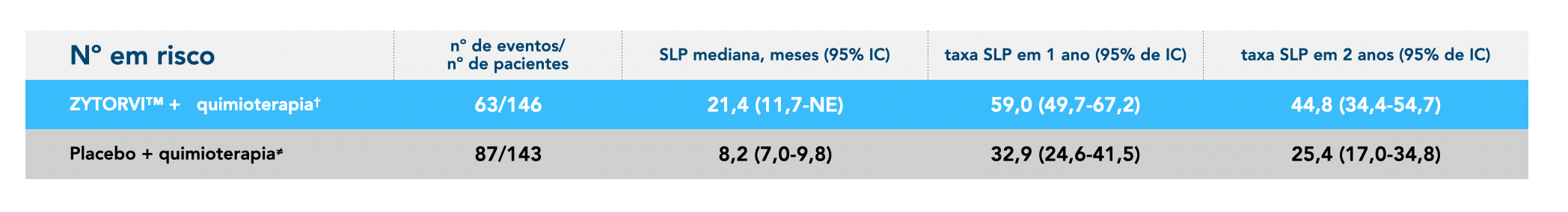
*The cutoff date for the interim analysis was June 8, 2021. 4,5
†Patients treated with Zytorvi ® + cisplatin and gemcitabine for up to six 21-day cycles followed by Zytorvi ® Based on Cox proportional hazards model stratified using n stratification factors in monotherapy¹ ‡Patients treated with placebo + cisplatin and gemcitabine for up to 6 21-day cycles followed by placebo monotherapy.¹ BIRC=blinded independent review committee; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; HR=hazard ratio; mPFS=median progression-free survival; RECIST/CARTS=response evaluation criteria in solid tumors; NE=not estimable.
References
-
ZYTORVI ® (toripalimab-tpzi) Prescribing Information.
-
ClinicalTrialsgov. 55 studies found for: recurrent, metastatic | nasopharyngeal carcinoma. Accessed September 28, 2023. https://clinicaltrials.gov/ct2/results?term=Recurrent%2C+Metastatic&cond=Nasopharyngeal+Carcinoma&age_v=&gndr=&type=&rslt=&phase=2&Search=Apply
-
Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 study. Nat Med. 2021;27(9):1536-1543. doi:10.1038/s41591-021-01444-0
-
Data on file. Coherus BioSciences, Inc. 2023
-
Mai HQ, Chen QY, Chen D, et al. Toripalimab + chemotherapy in advanced nasopharyngeal carcinoma: a JUPITER-02 randomized clinical trial. JAMA 2023; 330(20) :1961-1970.
-
Chen QY, Mai HQ, Chen D, et al. Four-year overall survival follow-up and dynamic EBV titer analysis of toripalimab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (r/m NPC). Abstract presented at: 2024 American Society of Clinical Oncology Annual Meeting. May 31-June 4, 2024; Chicago, IL.
-
Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol. 2021;39(7):704-712. doi:10.1200/JCO.20.02712
If you need a complete study, CLICK HERE to register your request