Polaris-02 study
STUDY DESIGN
The efficacy of Zytorvi® was evaluated in POLARIS-02, an open-label, multicenter, multi-cohort trial conducted in a single country.* The study included a total of 172 adult patients with unresectable or metastatic NPC who had received prior platinum-based chemotherapy for the treatment of recurrent or metastatic NPC or who had disease progression within 6 months of completing platinum-based chemotherapy administered as neoadjuvant, adjuvant, or definitive chemoradiation treatment for locally advanced disease.¹
Patients received ZYTORVI ® 3 mg/kg intravenously every 2 weeks until disease progression according to RECIST v1.1 or unacceptable toxicity. The main efficacy outcomes were confirmed ORR and DOR assessed by BIRC.
Eligibility Criteria⁷
-
Histologically or cytologically documented R/M NPC, refractory to prior standard chemotherapy.
-
Disease progression within 6 months after adjuvant chemotherapy or chemoradiotherapy.
-
Age 18 or over.
-
Measurable disease.
-
ECOG (Eastern Cooperative Oncology Group) performance status of 0 or 1.
-
Adequate organic function.
Exclusion Criteria⁷
-
Anticancer monoclonal antibody therapy within 4 weeks prior to starting treatment.
-
Any anticancer therapy within 2 weeks before starting treatment.
-
Previous treatment with ICI (Immune Checkpoint Inhibitors).
-
Systemic corticosteroid therapy within 7 days prior to initiation of treatment.
-
Known additional malignancies and active CNS metastases.
EFFECTIVENESS
Response was achieved in 21% of patients with ZYTORVI®1
Patients were heavily pretreated, with a median of 2 prior systemic therapies for disease R/M1.2*
Main outcomes of POLARIS-02: Confirmed ORR and DOR, as assessed by BIRC using RECIST v1.1.1
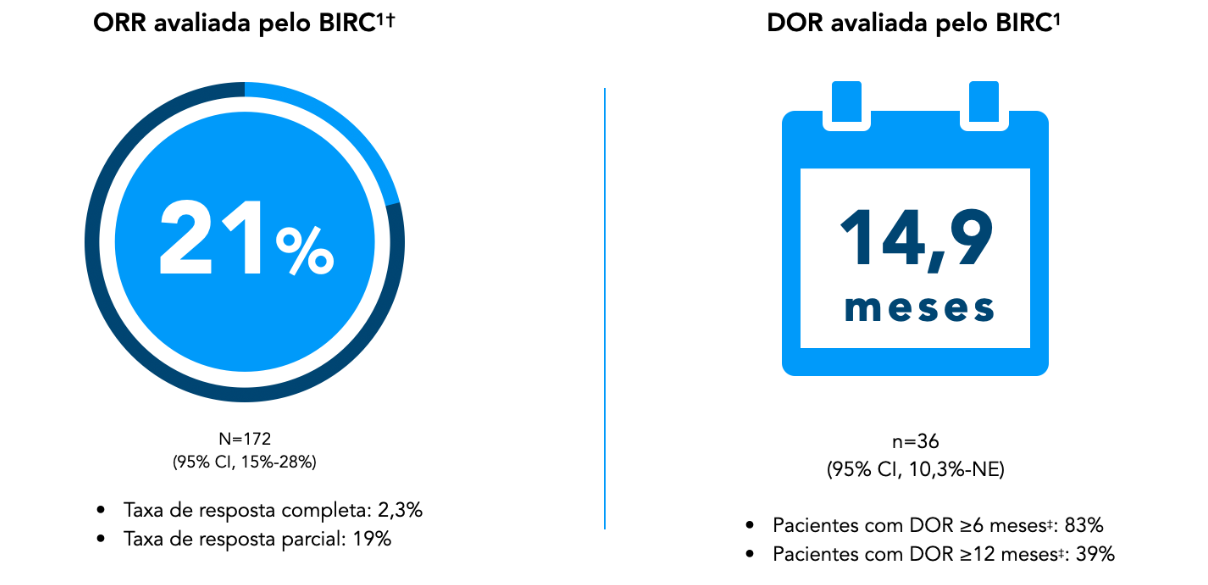
* Range: 1–13.1
† ORR confirmed and evaluated by BIRC.1
‡ Based on observed PAIN.1
BIRC = blinded independent review committee; CI = confidence interval; DOR = duration of response; NE = not estimable; R/M = recurrent/metastatic; ORR = overall response rate; RECIST = response evaluation criteria in solid tumors.
SECURITY
Adverse Reactions (≥10%) in Patients with Previously Treated Unresectable or Metastatic NPC Who Received ZYTORVI® in POLARIS-02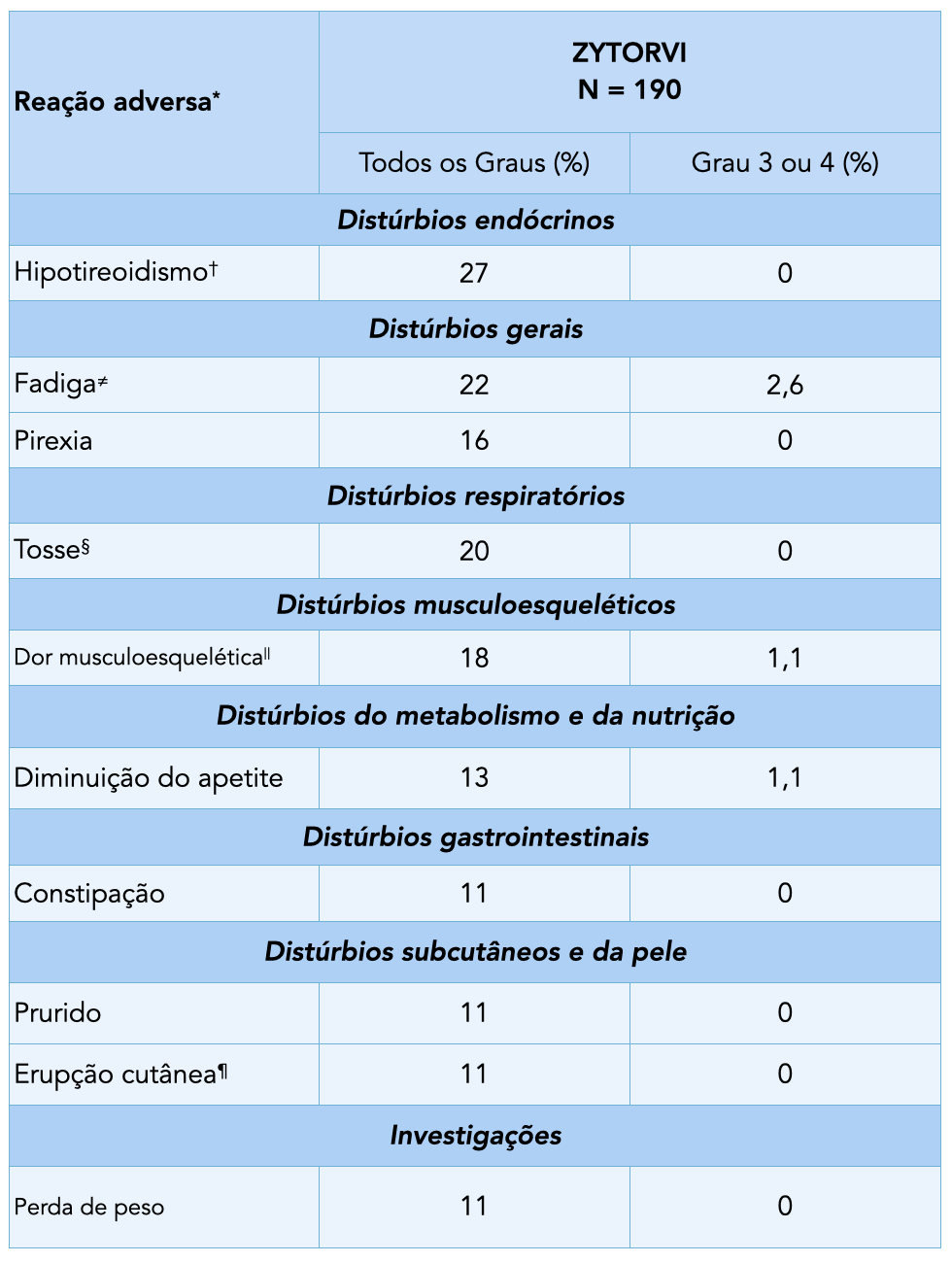
Serious and fatal adverse reactions
-
Serious adverse reactions occurred in 24% of patients receiving ZYTORVI®
-
Serious adverse drug reactions in ≥2% were pneumonia (4.7%), abnormal liver function (2.6%), and hyperbilirubinemia (2.1%)
-
-
Fatal adverse reactions occurred in 3.7% of patients receiving ZYTORVI®, including unspecified death (1.6%), tumor hemorrhage (0.5%), hepatic failure and thrombocytopenia (0.5%), hyponatremia (0.5%), and sudden death (0.5%).
Dosage discontinuations and interruptions
-
Permanent discontinuation of ZYTORVI® due to an adverse reaction occurred in 9% of patients
-
Adverse reactions resulting in permanent discontinuation of ZYTORVI® in ≥1% of patients were pneumonia (1.1%), abnormal liver function (1.1%), and hyperbilirubinemia (1.1%).
-
-
Dosage interruptions due to an adverse reaction occurred in 23% of patients
-
Adverse reactions requiring dose interruption in ≥1% were pneumonia (2.1%), thrombocytopenia (2.1%), fatigue (1.6%), hyperbilirubinemia (1.6%), anemia (1.1%), decreased appetite (1.1%), abnormal liver function (1.1%), hypothyroidism (1.1%), and pneumonitis (1.1%)
-
*Toxicity was graded according to NCI CTCAEv4.03.1
†Includes hypothyroidism, thyroiditis, decreased triiodothyronine, and decreased free triiodothyronine.1
‡Includes fatigue and asthenia.1
§Includes cough and productive cough.1
‖ Includes musculoskeletal pain and myalgia.1
¶Includes allergic dermatitis, eczema, and rash.1*The POLARIS-02 study was conducted at 17 sites in China.²
† Tumor response assessments were performed every 8 weeks for the first year and every 12 weeks thereafter.1
Abbreviations
-
BIRC: Independent Blind Review Committee
-
PAIN: Duration of response
-
ORR: Overall Response Rate
-
R/M NPC: Recurrent locally advanced/metastatic nasopharyngeal cancer
-
RECIST: Response evaluation criteria in solid tumors
References
-
ZYTORVI® (toripalimab-tpzi) Prescribing Information.
-
ClinicalTrialsgov. 55 studies found for: recurrent, metastatic | nasopharyngeal carcinoma. Accessed September 28, 2023. https://clinicaltrials.gov/ct2/results?term=Recurrent%2C+Metastatic&cond=Nasopharyngeal+Carcinoma&age_v=&gndr=&type=&rslt=&phase=2&Search=Apply
-
Mai HQ, Chen QY, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 study. Nat Med. 2021;27(9):1536-1543. doi:10.1038/s41591-021-01444-0
-
Data on file. Coherus BioSciences, Inc. 2023
-
Mai HQ, Chen QY, Chen D, et al. Toripalimab + chemotherapy in advanced nasopharyngeal carcinoma: a JUPITER-02 randomized clinical trial. JAMA 2023; 330(20):1961-1970.
-
Chen QY, Mai HQ, Chen D, et al. Four-year overall survival follow-up and dynamic EBV titer analysis of toripalimab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (r/m NPC). Abstract presented at: 2024 American Society of Clinical Oncology Annual Meeting. May 31–June 4, 2024; Chicago, IL.
-
Wang FH, Wei XL, Feng J, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02). J Clin Oncol. 2021;39(7):704-712. doi:10.1200/JCO.20.02712
If you need a complete study, CLICK HERE to register your request