Jupiter-02 Efficacy: final analysis (secondary outcome) and 4-year post-hoc analysis
Final analysis (secondary outcome): Zytorvi® + chemotherapy significantly reduced the risk of progression 1.4†
Overall survival not reached with Zytorvi® + chemotherapy (95%CI, 38.7-NE) vs 33.7 months with placebo + chemotherapy (95%CI, 27.0-44.2); HR=0.63 (95% CI, 0.45-0.89); P=0.0083≠
-
37% reduction in risk of death
-
Number of events was 57/146 (39%) with Zytorvi® + chemotherapy vs 76/173 (53%) with placebo + chemotherapy
4-year post-hoc analysis 6§
Post-hoc analysis of an exploratory nature performed after the final analysis specified by protocol 6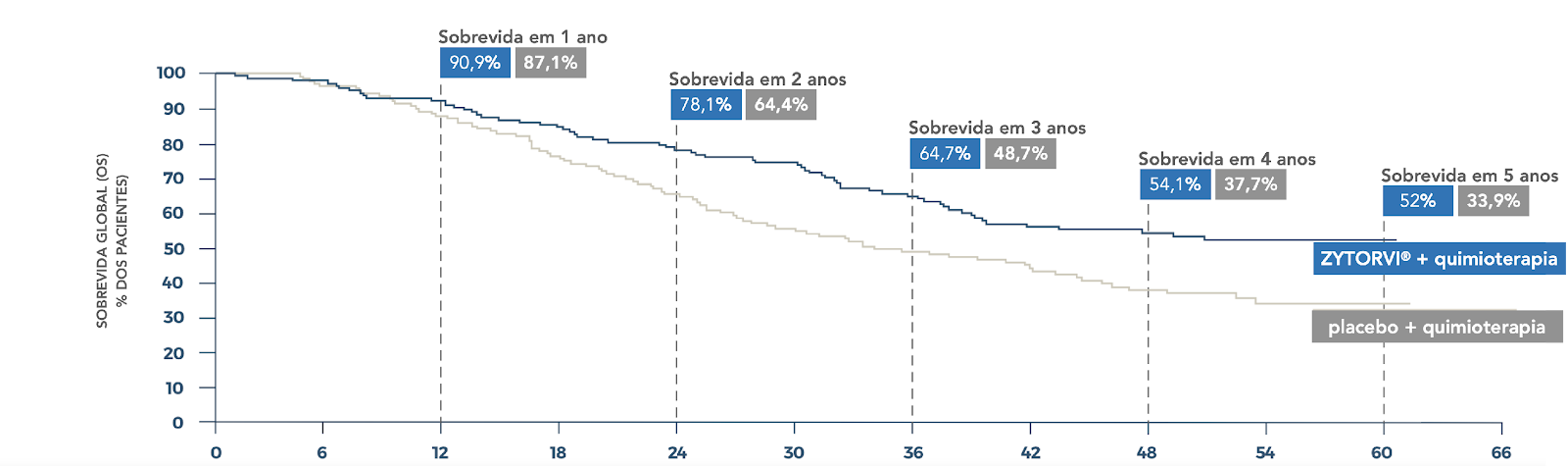
-
Median overall survival not reached with Zytorvi ® + chemotherapy (95%CI, 38.8-NE) vs 33.7 months with placebo + chemotherapy (95%CI, 26.7-44.2); HR=0.61 (95% CI, 0.44-0.85); median follow-up was 36.8 months (range 0.2-6.11 months) 6
-
39% reduction in risk of death
†Patients treated with Zytorvi® + cisplatin and gemcitabine for up to six cycles, followed by Zytorvi® monotherapy or placebo monotherapy.
†The cutoff date for the final analysis was November 18, 2022. The median follow-up was 36 months .
≠two-sided P-value based on stratified log-rank test, compared with an alpha threshold of 0.049995 1
§After the final OS analysis was conducted as specified by the study protocol, surviving patients were re-consented for long-term survival follow-up.
OS=Overall survival; mPFS=Median Progression-Free Survival; NE=not estimable.